The Lustgarten StoryThe Lustgarten Foundation Changed Everything
When the Lustgarten Foundation was established, pancreatic cancer was considered an orphan disease with very little money going into research and virtually no long-term treatment options. Lustgarten changed all that. Today, there are thousands of researchers working to improve patient outcomes and to discover breakthroughs in pancreatic cancer treatments.
Learn about Marc Lustgarten and how his diagnosis and experience launched a game-changing Foundation with a critical mission.
Read MoreThe Strategy
The Lustgarten Foundation exists to transform pancreatic cancer into a curable disease. We are committed to stretching the boundaries of science and centering our research programs on the areas with the greatest potential for impacting patients, including earlier detection, therapeutics and personalized medicine.
Read MoreYour Impact
As the world’s largest private funder of pancreatic cancer research, we believe research is fundamental to giving patients and their families more hope and more time. The Foundation’s funded science has been a driving force in every major advancement in pancreatic cancer research. Thanks to donors like you and the scientists whose critical work we support, pancreatic cancer research is now advancing faster than ever before.
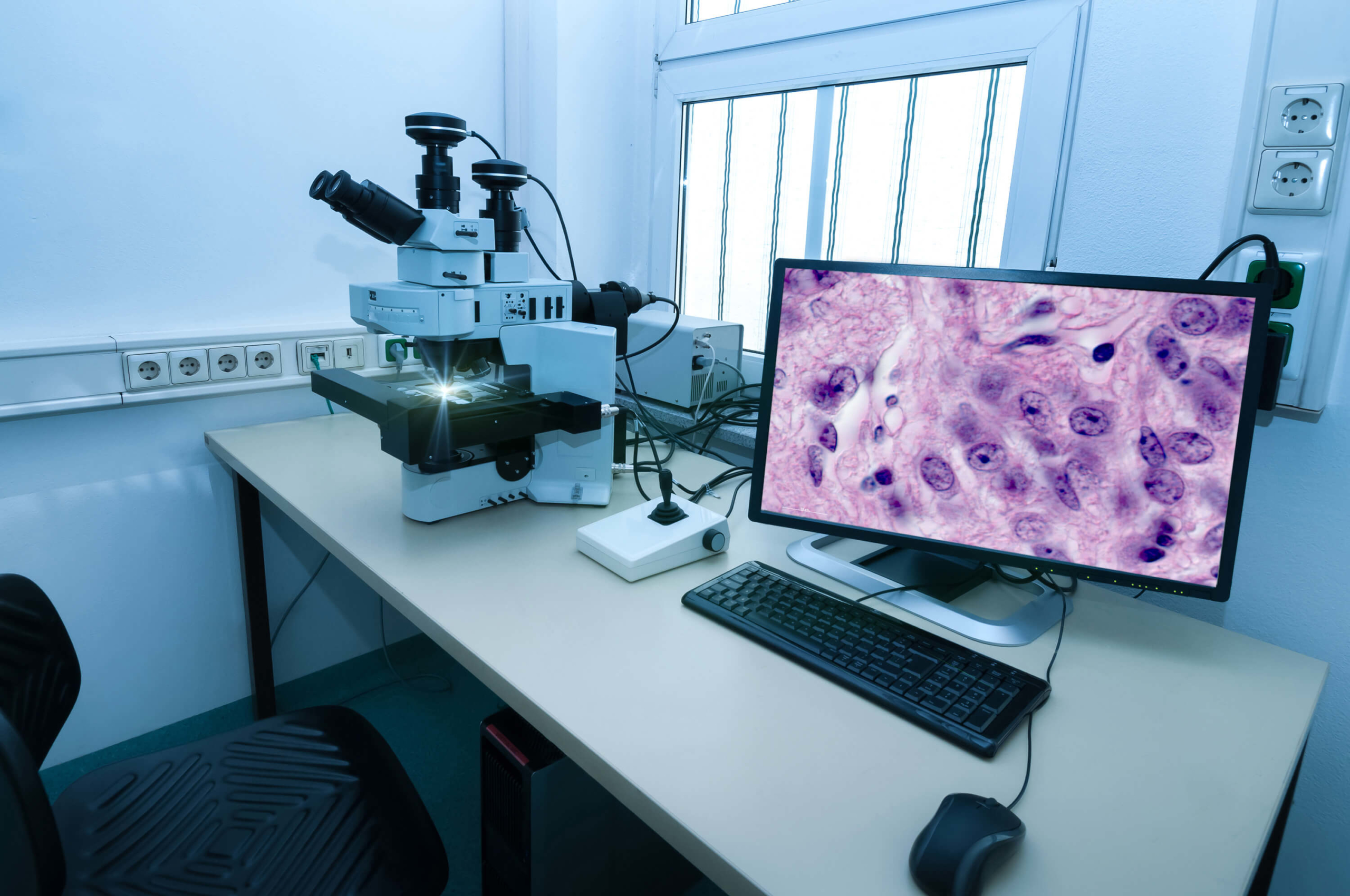
Read MoreLustgarten Labs
The Foundation is paving the way toward a cure with our collaborative network of labs dedicated to pancreatic cancer research. Every day, scientists are gaining momentum in advancing early detection methods, discovering new treatments and improving patient outcomes.
Read MoreBoard and Leadership
We couldn’t fulfill our mission without the support of our committed board of directors, corporate and scientific advisory boards, and passionate staff and volunteers who work tirelessly to help patients and support our researchers.
Read More100% of your donation funds pancreatic cancer research.